Safety
Argentyn 23® is manufactured in an NSF GMP-Registered manufacturing facility with a laser focus on purity, efficacy and safety. At 23 ppm, Argentyn 23 falls below the silver daily Oral Silver Reference Dose (RfD) of 350 mcg (micrograms) as established by the EPA.†
Purity
The purity of the silver, the water and the formulation affect the safety of silver products. The purest products are the safest; impurities or additional ingredients can negatively impact the safety profiles of silver dietary supplement products.
Argentyn 23® Bio-Active Silver HydrosolTM contains just two ingredients: 99.999% pure silver and water that meets USP-NF standards for pharmaceutical-grade purified water. It delivers only naturally occurring silver nanoparticles and bio-active silver ions, and is stored in amber glass to protect its purity.
Argentyn 23 Bio-Active Silver Hydrosol is free of proteins, salts, and other compounds and therefore poses no health hazard when used as directed.* However other colloidal silvers may contain these impurities, therefore, decreasing efficacy and increasing the risk of causing Argyria.
Toxicity
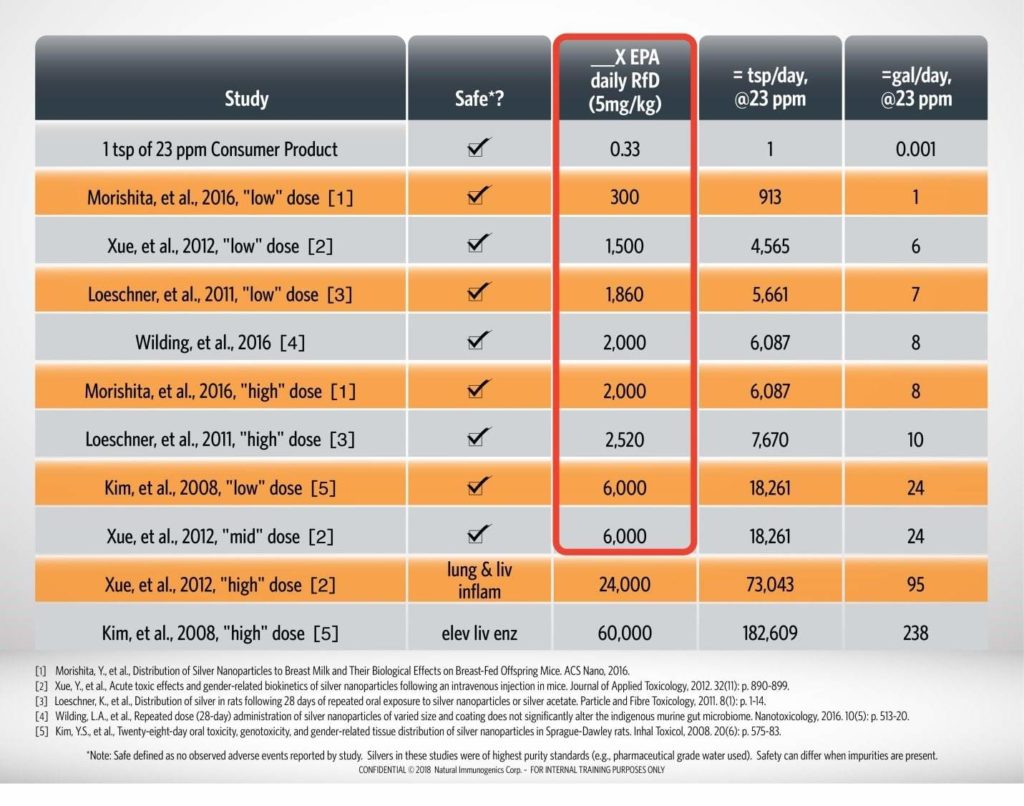
The potential for silver nanoparticles to have acute or chronic toxicity has been researched in numerous peer-reviewed in vivo studies. Adverse effects were not observed, even at 6,000X the RfD. With Argentyn 23, consuming that amount would require consuming nearly 24 gallons of water, a volume of liquid that would pose a health hazard from the water alone.
A summary of peer-reviewed literature studies on ionic silver and colloidal silver (silver nanoparticles) is presented in the table below.1-5 No observed adverse events are reported by two studies at 6,000X the EPA daily reference dose (RfD) of 5 micrograms of silver per kilogram of bodyweight. It is critical to note that all literature studies used high purity water, high purity silver materials, and endotoxin free materials. The purity of Argentyn 23 exceeds the standards used in these studies, thereby allowing interpretation of this literature with confidence. The presence of other ingredients in the formulations or other contaminants can dramatically impact the safety profile of other colloidal silver products.
Bio-Active Silver Hydrosol has not been reported to cause a single case of argyria. Developing argyria has typically been reported when silver exposure exceeds a lifetime 10-gram exposure,6 generally through extensive abrasive dermal contact with silver salts or silver compounds, long-term exposure through inhalation of silver powder, or consumption of extreme amounts, as might be ingested with unregulated, homemade colloidal silver.
Efficacy*
Particle size greatly impacts the efficacy of silver products. Larger particles provide less surface area, which reduces bioavailability. Smaller silver particles supply more surface area and, therefore, have greater potential for releasing beneficial bio-active silver ions into the system during the short time the silver remains in the body.

Argentyn 23 has an unprecedented particle size as small as 0.8 nm (nanometers). This equates to >98% bioavailability and therefore extremely little waste as almost all of the silver is able to provide immune support for the body.
Products with high parts per million (PPM) concentrations should be avoided as those products generally contain <10% bioavailable silver and greatly exceed the EPA oral Daily Reference Dose (RfD)† for silver of 350 mcg.
About
Silver is naturally occurring in many places, and it is ingested in drinking water and foods, including wheat, mushrooms, fish, chocolate and mammalian milk.7According to a study conducted in the United Kingdom, the average adult intake from a normal diet is 27 micrograms daily.8
Argentyn 23 Bio-Active Silver Hydrosol is safe* when following label directions or as directed by a healthcare professional. Argentyn 23 delivers 23 ppm of 99.999% pure silver, in a safe and effective immune support dosage of 1 tsp/day. For short-term support, multiple doses can be taken in a day.
Argentyn 23 Bio-Active Silver Hydrosol is the only professional silver supplement to have received a statement of safety from Dr. Dana Flavin; Founder and Executive Director; former Science Assistant to the Associate Bureau Director, Division of Toxicology, US FDA, Washington DC.
Previous: Choosing the Most Effective* Silver
References
- Morishita Y, Yoshioka Y, et al., Distribution of silver nanoparticles to breast milk and their biological effects on breast-fed offspring mice. ACS Nano, 2016.
- Xue Y, Zhang S, et al., Acute toxic effects and gender-related biokinetics of silver nanoparticles following an intravenous injection in mice. Journal of Applied Toxicology, 2012. 32(11): p. 890-899.
- Loeschner K, Hadrup N, et al., Distribution of silver in rats following 28 days of repeated oral exposure to silver nanoparticles or silver acetate. Particle and Fibre Toxicology, 2011. 8(1): p. 1-14.
- Wilding LA, Bassis CM, et al., Repeated dose (28-day) administration of silver nanoparticles of varied size and coating does not significantly alter the indigenous murine gut microbiome. Nanotoxicology, 2016. 10(5): p. 513-20.
- Kim YS, Kim JS, et al., Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in sprague-dawley rats. Inhal Toxicol, 2008. 20(6): p. 575-83.
- Zumwalde R, Kuempel E, et al., External review draft – current intelligence bulletin: Health effects of occupational exposure to silver nanomaterials., U.S. Department of Health and Human Services CfDCaP, National Institute for Occupational Safety and Health, Editor. 2015, NIOSH: Cincinnati, OH.
- Millour S, Noël L, et al., Strontium, silver, tin, iron, tellurium, gallium, germanium, barium and vanadium levels in foodstuffs from the second french total diet study. Journal of Food Composition and Analysis, 2012. 25(2): p. 108-129.
- Hamilton EI,Minski MJ, Abundance of the chemical elements in man’s diet and possible relations with environmental factors. Science of The Total Environment, 1973. 1(4): p. 375-394.